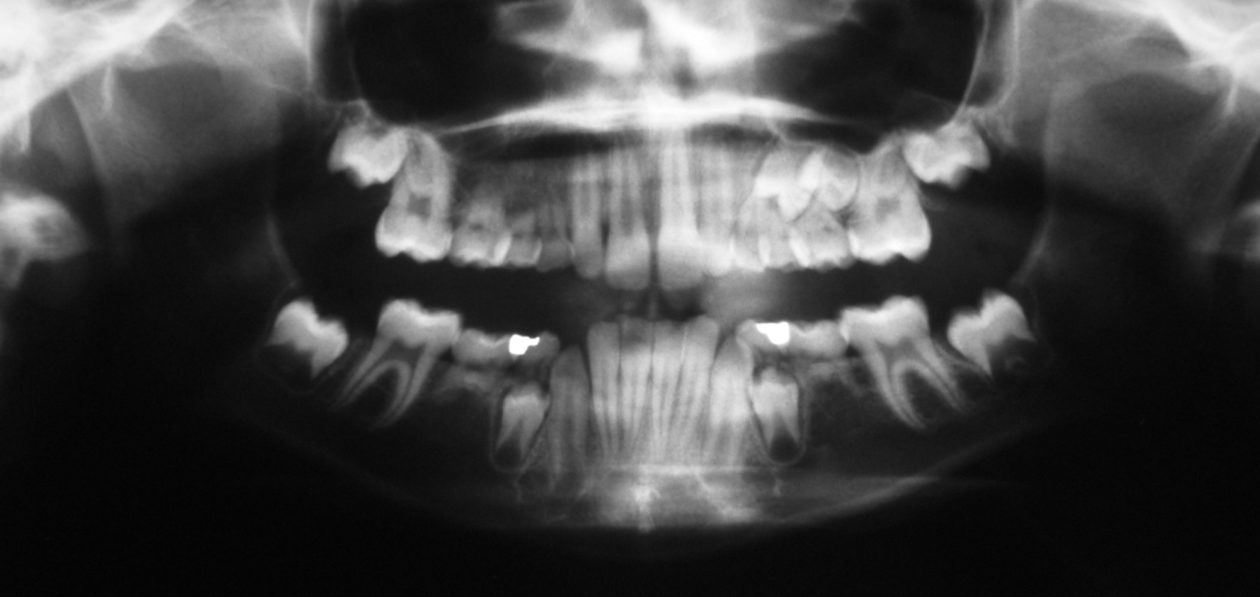
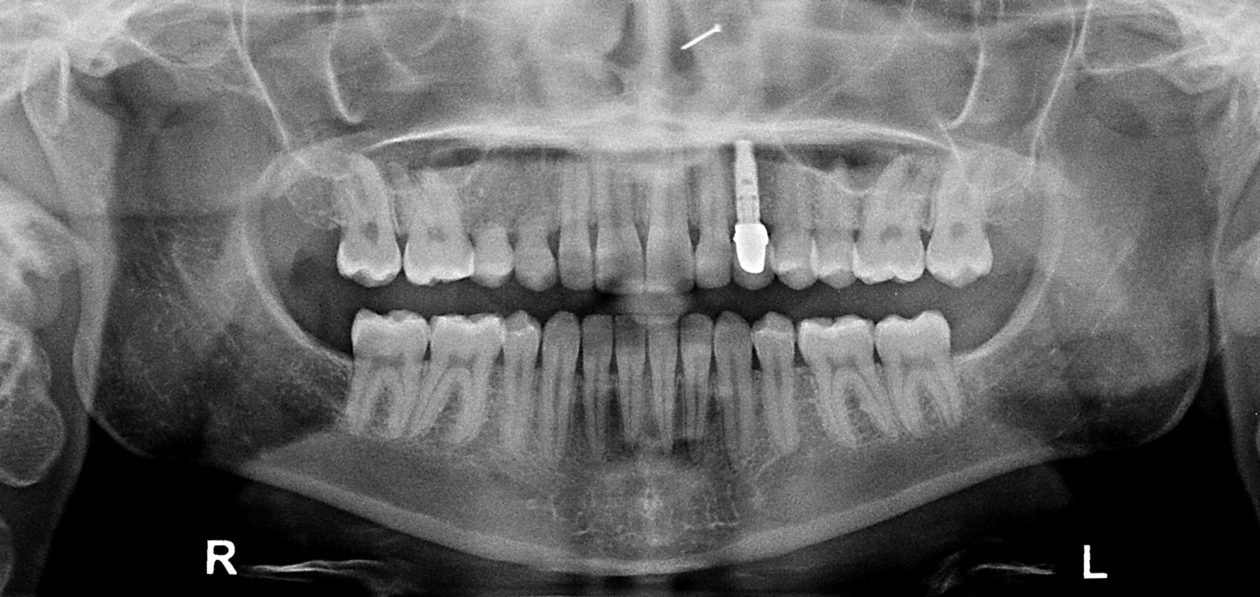
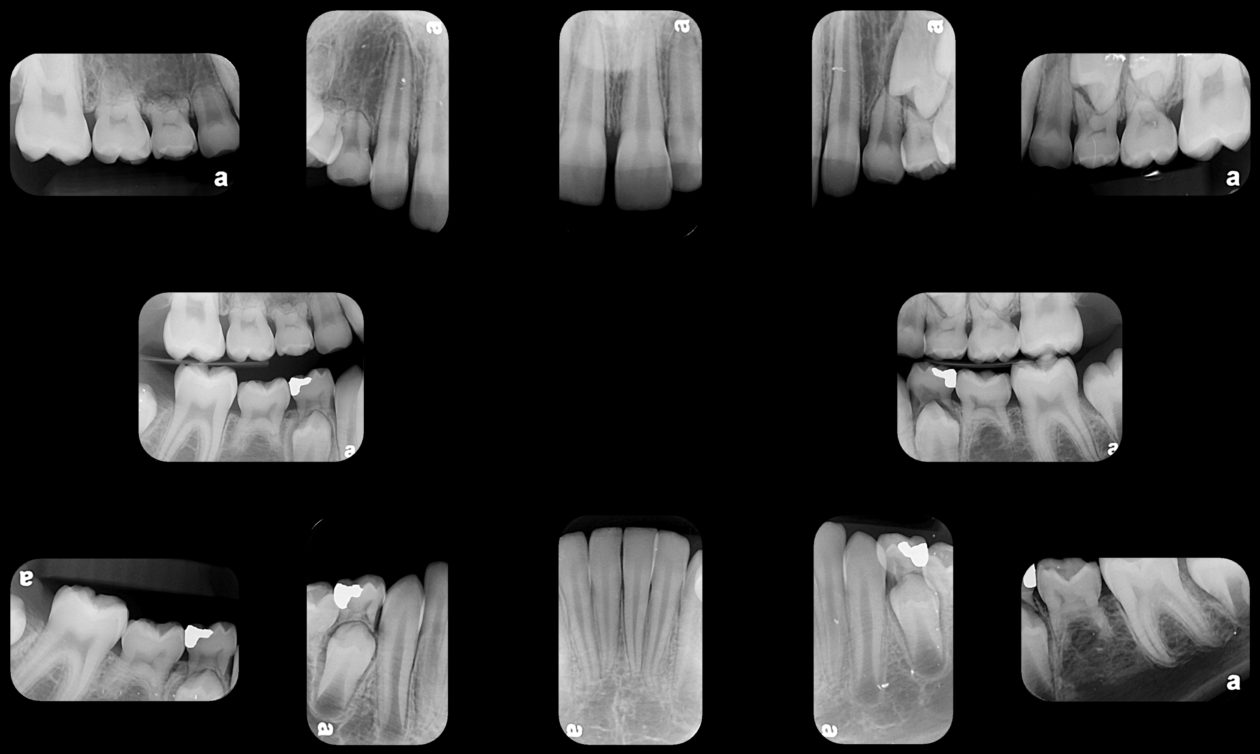
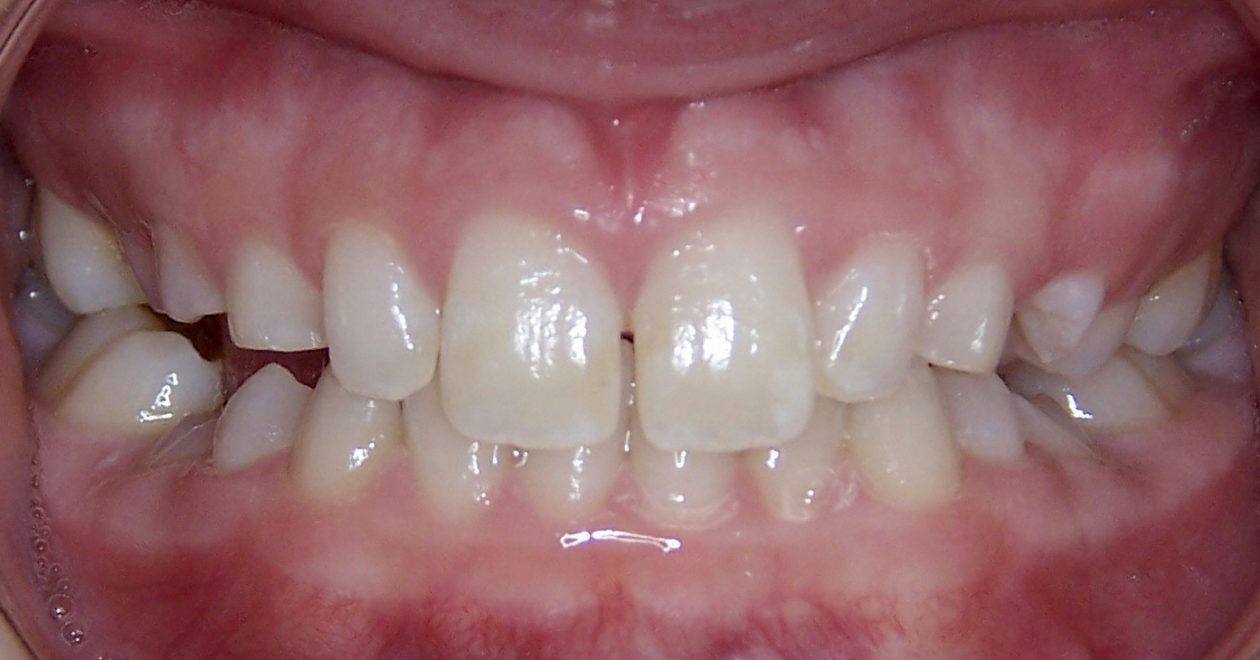
Phase VI: Oral Surgery Treatment
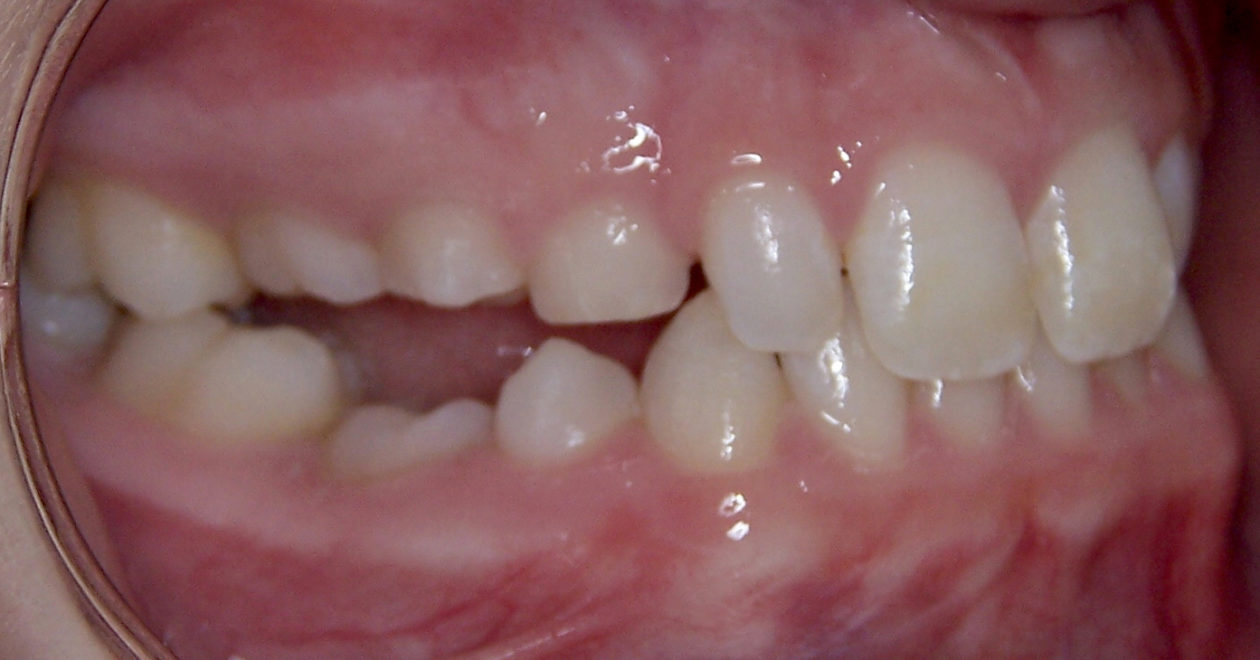
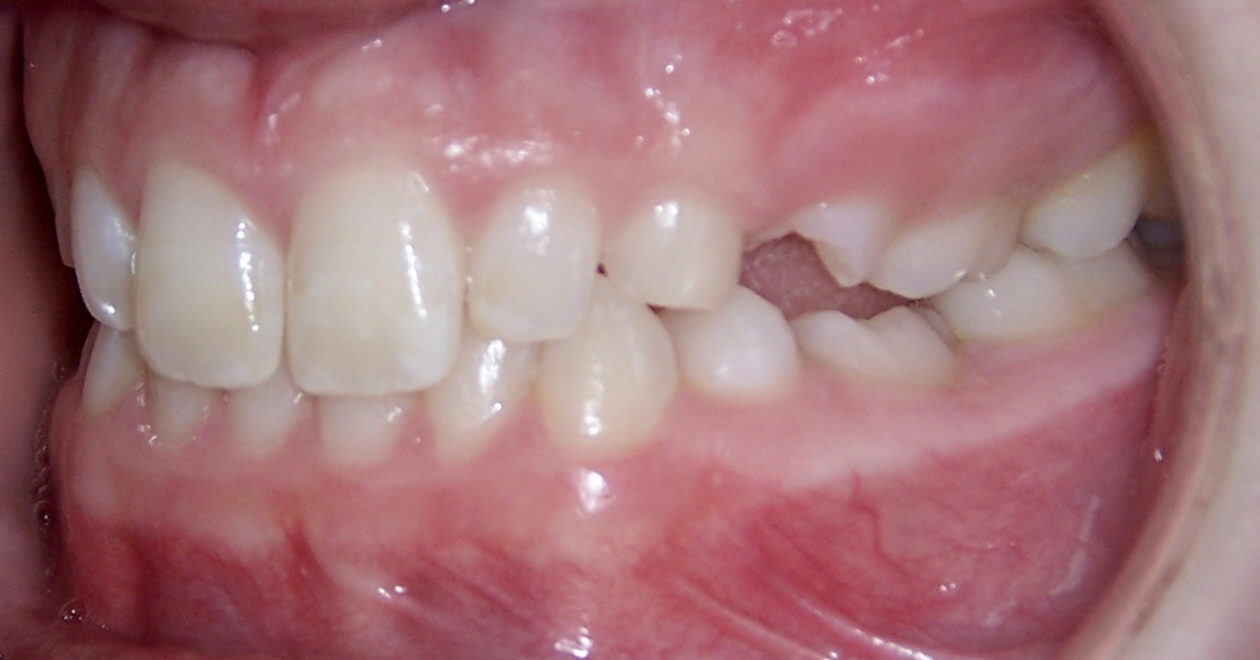
After orthodontic retreatment was completed retainers incorporating a temporary tooth no. 11 were created. Cone beam imaging of implant site no. 11 showed the need for bone grafting to fill out the width of the implant site. A mixture of Geistlich Bio-Oss and Zimmer Puros grafting material was placed utilizing Baxter Tisseel fibrin sealant and a Puros pericardium membrane and allowed to heal for four months. Upon re-evaluation the facial bone thickness was adequate but the lingual bone contour had an apical “crater.” A second bone graft on the lingual side was completed with Geistlich Bio-Oss and allowed to heal another five months. Once adequate bone contour was confirmed a Zimmer Eztetic implant was placed using a flapless technique, a healing abutment was placed flush to the tissue surface, and the patient continued to wear her retainer.
Phase VII: Restorative Treatment
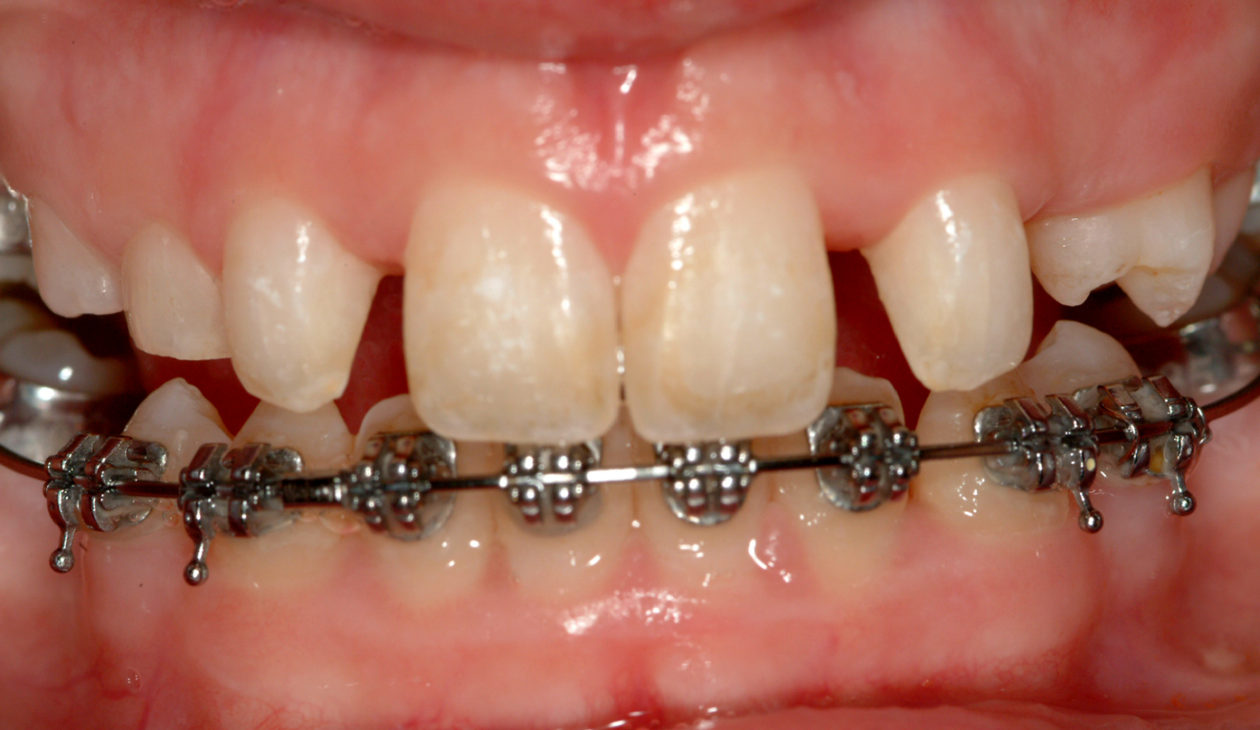
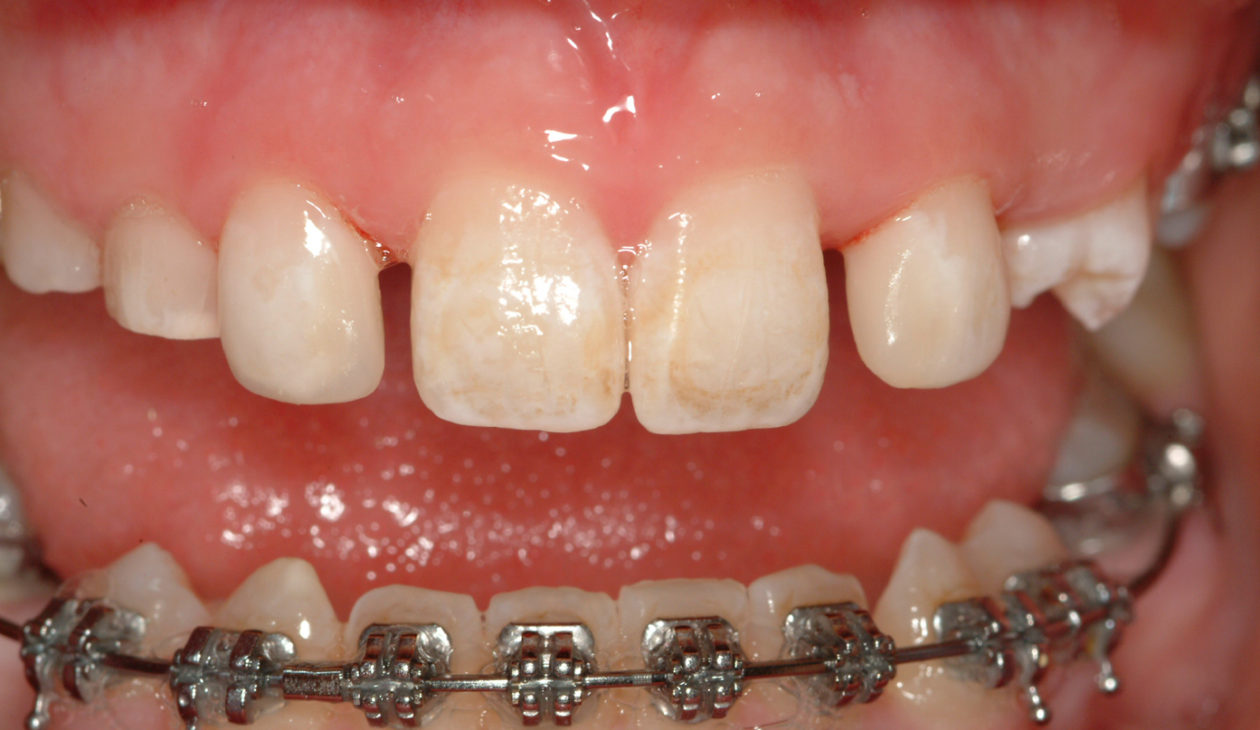
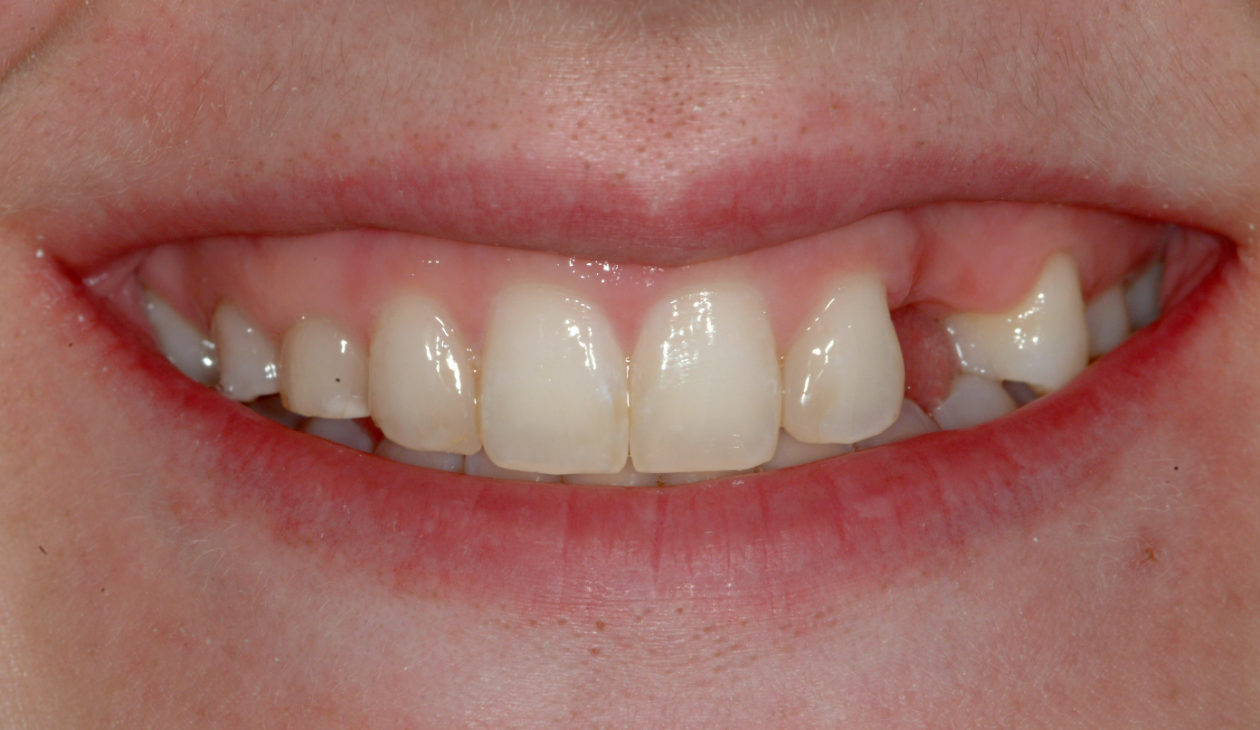
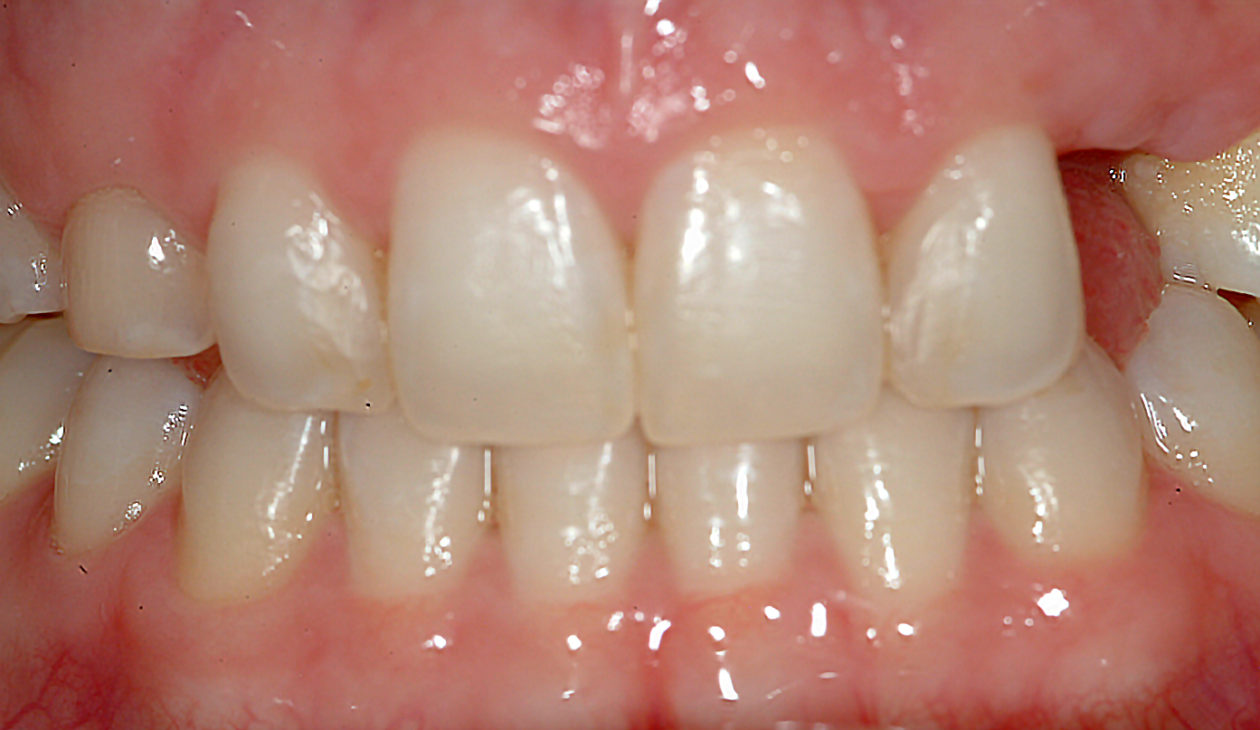
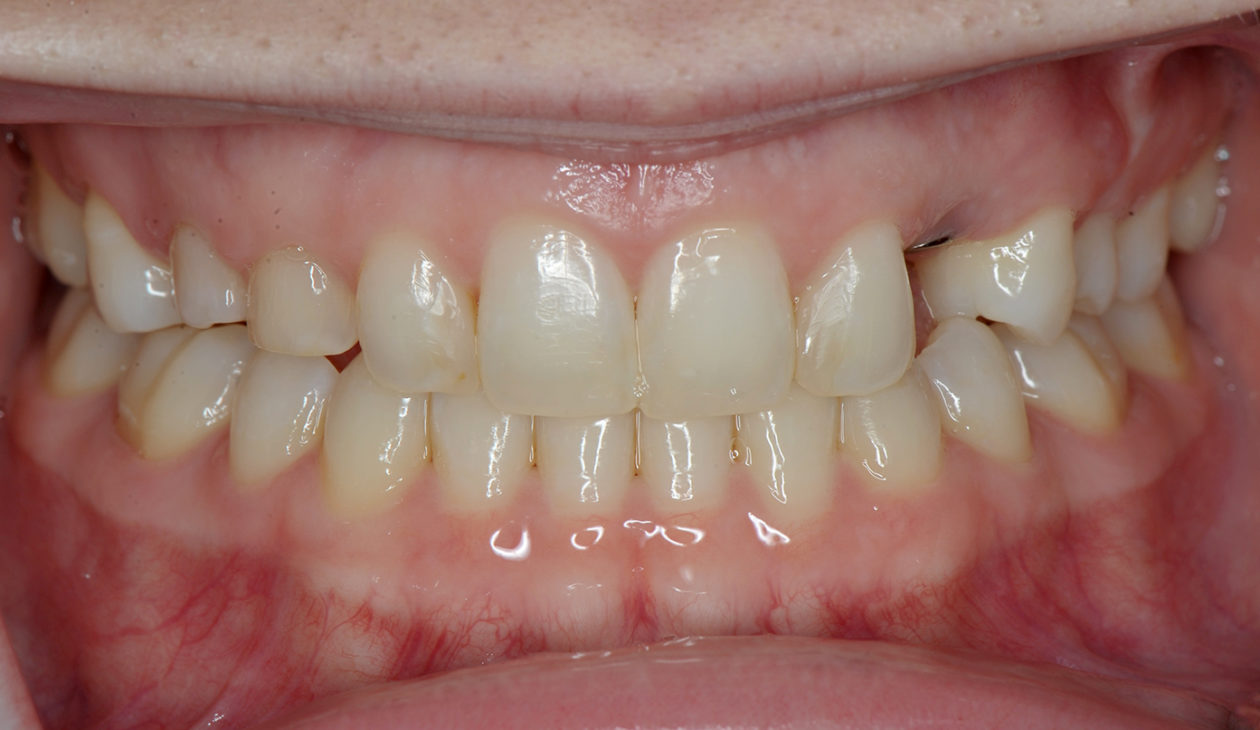
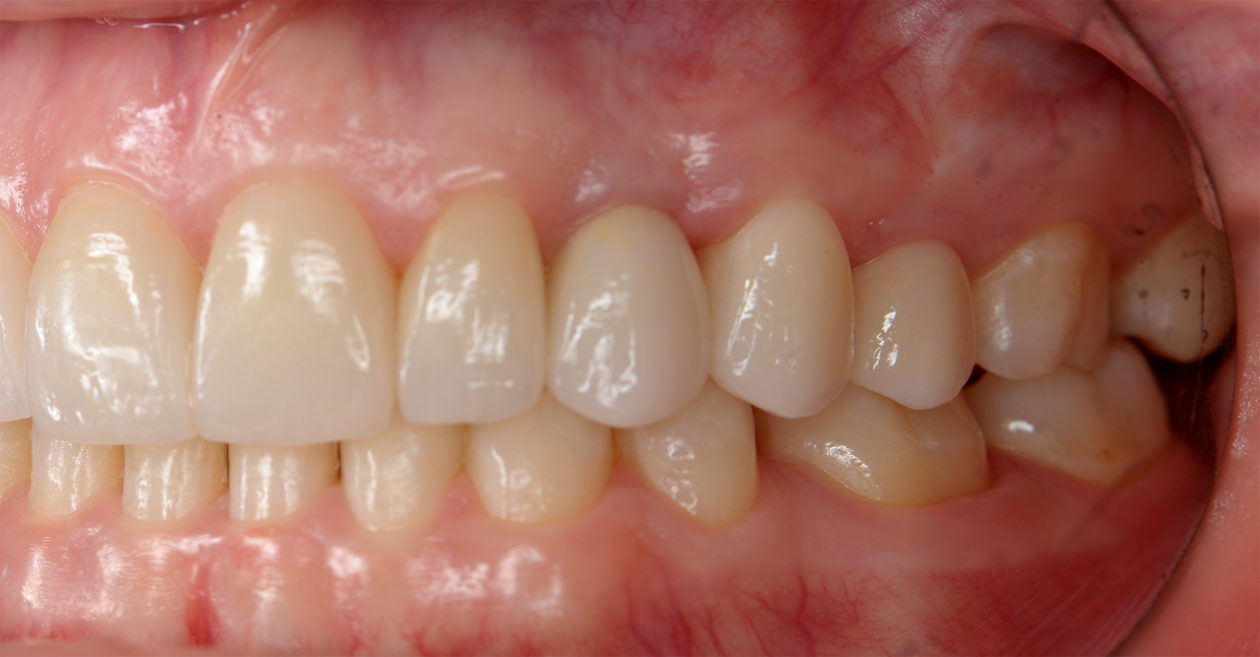
Once the site no. 11 implant was integrated a diagnostic wax-up was completed for the final restorative phase and a new intraoral “mock up” was made and tried in for patient and parent approval. Preparation of the teeth was broken up into gross prep and fine prep appointments so that any minor adjustments could be achieved. At the gross prep appointment an impression for the zirconia implant abutment was obtained and laser soft tissue recontouring was completed on teeth nos. C, 7, and 12. Three weeks later the final veneer preparations on teeth nos. B, C, 7, 8, 9, 10, 12, and 13 were completed. The site no. 11 zirconia abutment was seated. Impressions, final shade photos, and models of the provisional restorations were obtained.
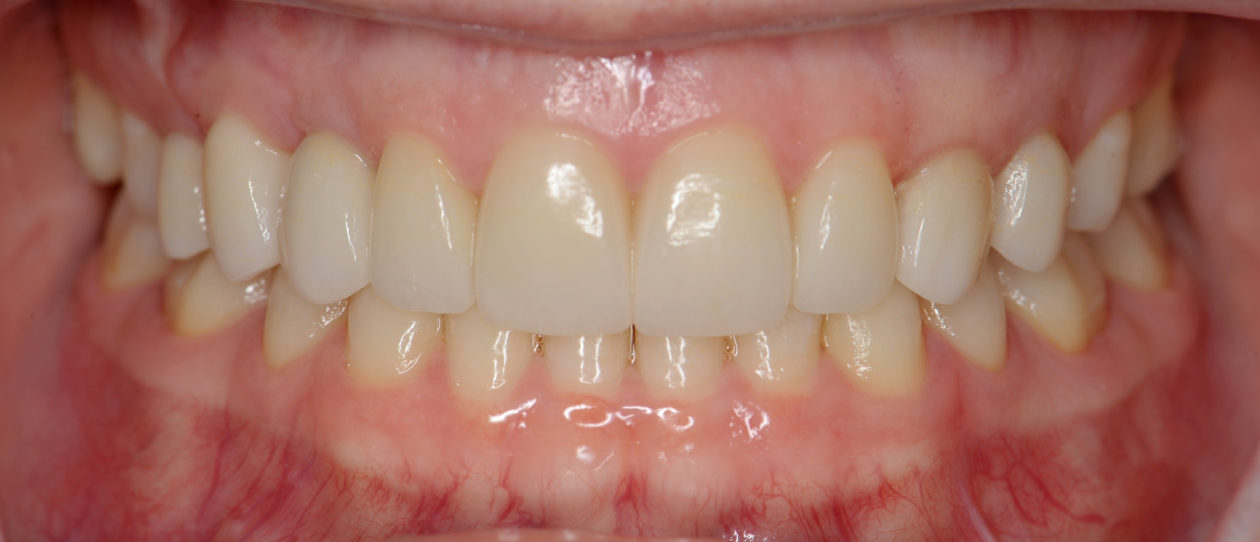
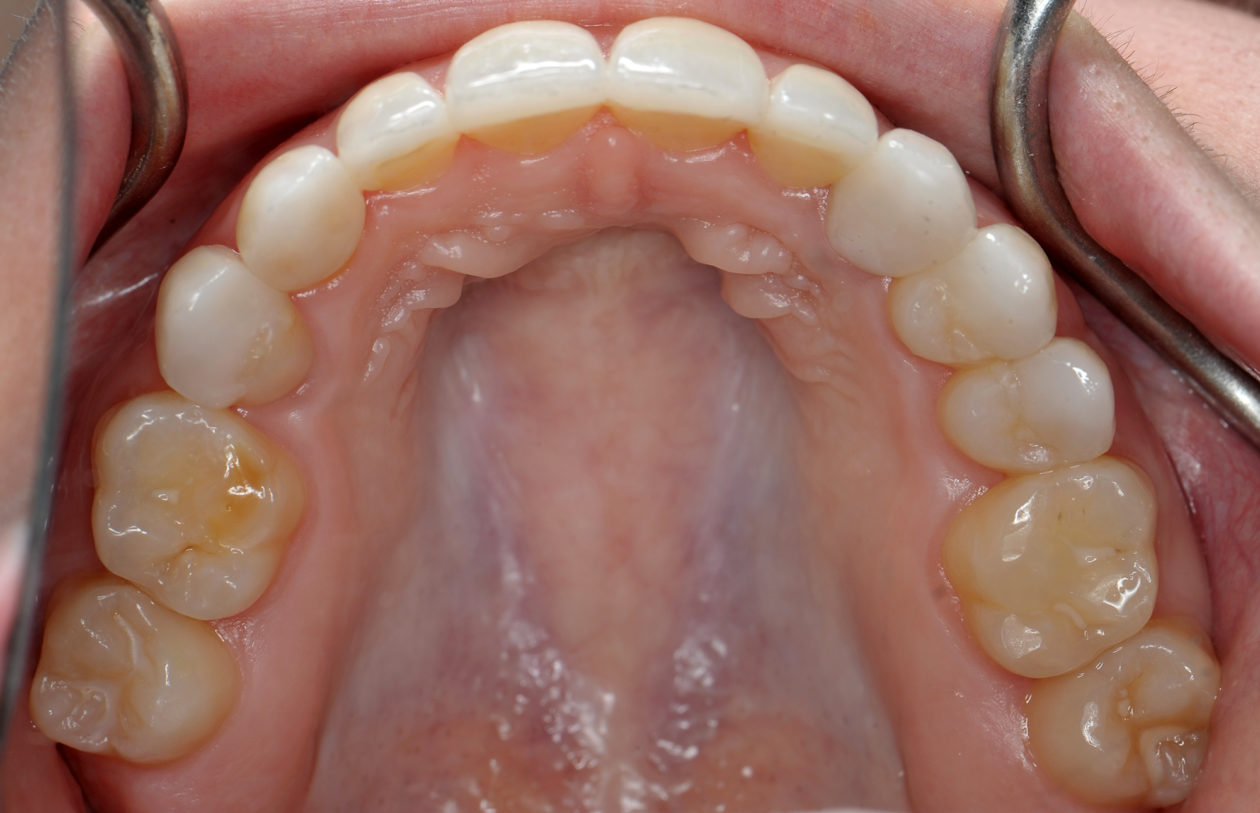
Four weeks later the final Ivoclar e-Max restorations for teeth nos. B, C, 7, 8, 9, 10, 12, and 13 were cemented with 3M RelyX Veneer Cement. The final Ivoclar e-Max restoration for implant site no. 11 was cemented with Premier Implant Cement.
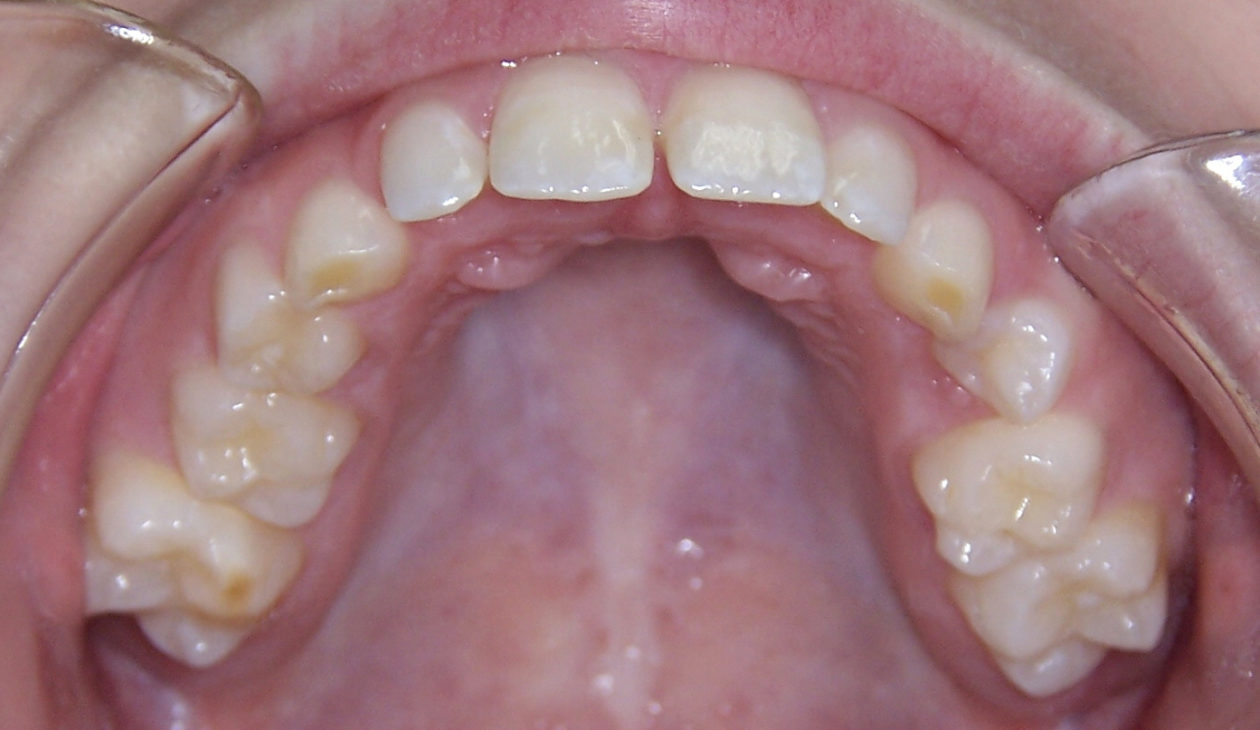
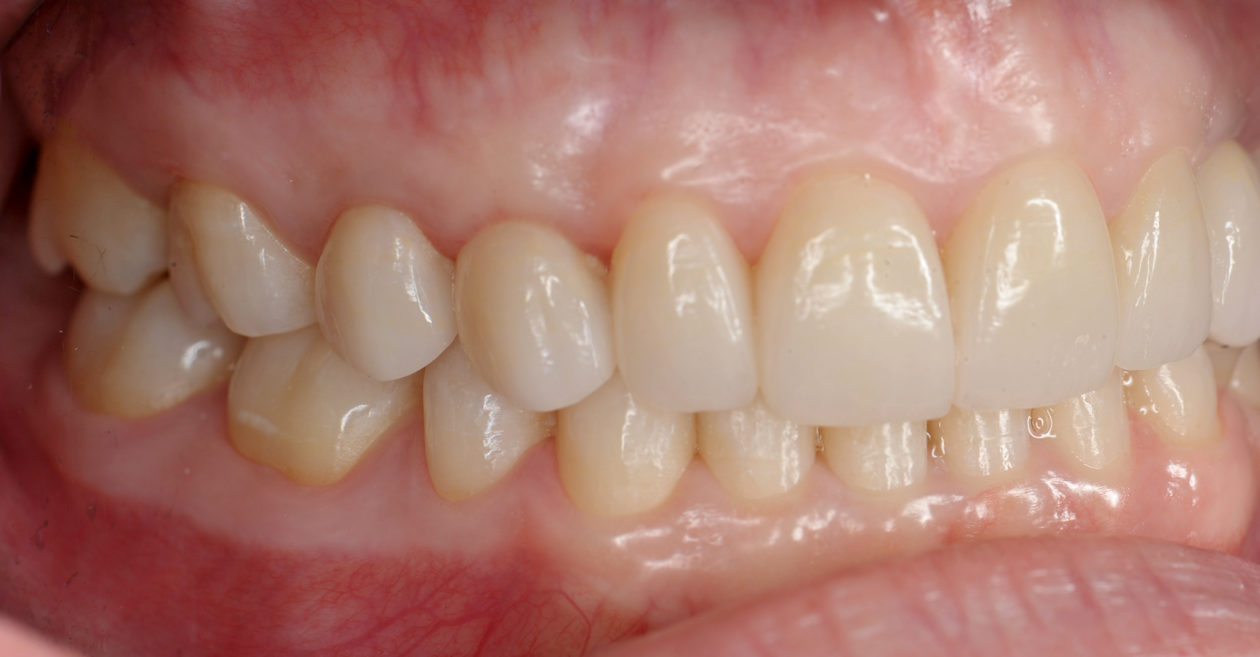
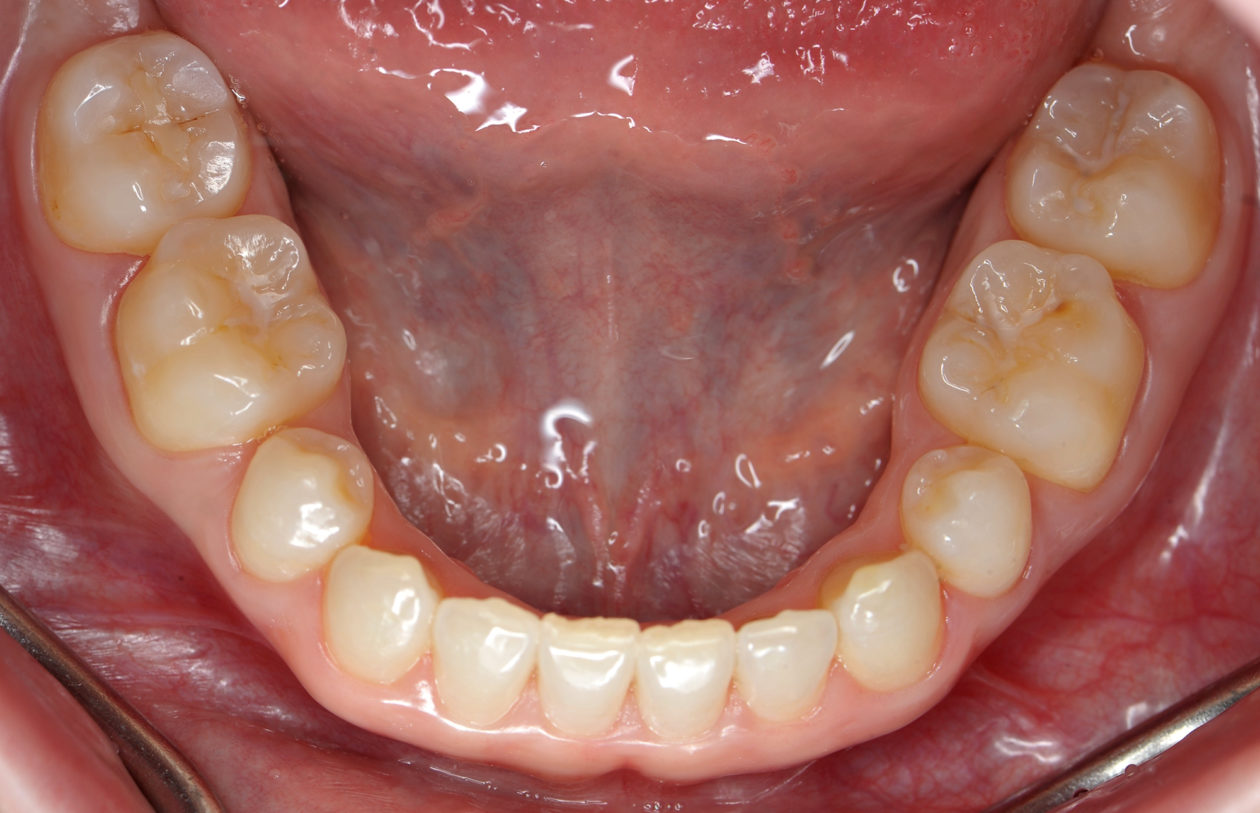
The occlusion was finalized and a progressive group function was established on teeth nos. 3, B, and C.
Phase VIII: Maintenance
The patient received a maxillary nightguard/retainer with a shallow anterior guidance and is being seen on a regular six-month recall schedule. Patient is maintaining excellent oral hygiene, is using an OTC fluoride mouthwash, and is wearing her nightguard. Patient is also maintaining orthodontic and oral surgery recalls.
The occlusion and the retained primary teeth nos. B and C are being closely monitored at recall appointments and to this date show no signs of being lost.